Pastry science #3 – The properties of sugar (Part II)
A few weeks ago I started discussing the main characteristics of sugars (and here is a classification of sugars).
All sugars have these characteristics in common:
- They are sweet (duh!)
- They give structure
- They form crystals
- They melt
- They absorb humidity
- They preserve
- They lower the freezing point and increase the boiling point of water
- They facilitate the fermentation
- They caramelize and are subject to the Maillard reaction
I dealt with the first 4 points in my previous article. For the remaining characteristics, including the Maillard reaction, read on…
5. Hygroscopicity (uh?)
All sugars are hygroscopic, albeit at different levels: this means that they have a tendency to “grab” water/humidity and keep it as long as they can. That is exactly why powdered sugar on top a poundcake “disappears” or “gets wet” after a while: it has just absorbed humidity from the air.
Of course, absorbing humidity is a great way to keep our cakes soft and moist. No wonder then that products like super fudge brownies can contain a big quantity of sugar: it is fundamental to keep them soft even hours (or days) after baking.
The most hygroscopic sugar is fructose, which can keep baked goods moist longer than other sugars. The same applies to the syrups containing fructose, like agave syrup and honey. The different levels of hygroscopicity also mean that we cannot simply replace 100% of the sugar in a recipe with honey, because the final consistency will be altered, too soft or spongy.
6. Preservation
Bacteria thrive in water. Mold, for example, cannot grow in a waterless environment. The sugar contained in products such as candied fruits or fruit preserves (which is usually a lot!) can attract all the water, leaving the bacteria empty-handed! No bacteria means that the final product can be stored for longer without deteriorating.
7. Impact on the freezing and boiling point of water
Water freezes at 0°C and boils at 100°C. Adding sugar to water will make it freeze at negative temperatures and boil at temperatures above 100°C. The impact on the freezing point is particularly useful in ice cream making (we want a creamy product, not a block of ice), while the impact on the boiling point is more important for candy making.
8. Fermentability
Sugars are able to trigger the fermentation in products: this is particularly desired in kneaded doughs, which contain yeast. Yeast makes the dough rise because it is able to produce carbon dioxide by “digesting” (metabolising) glucose.
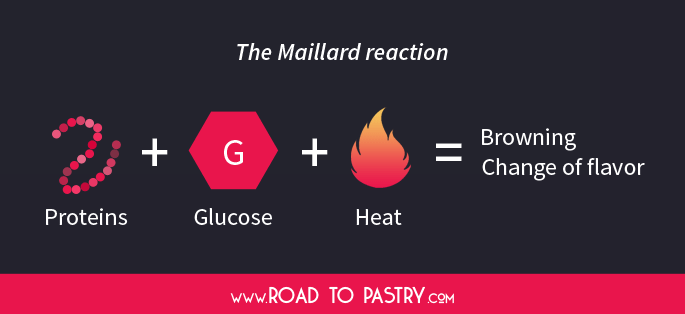
9. The one and only “Maillard reaction” + Caramelization
The Maillard reaction is a process that happens when proteins and the so-called “reducing sugars” interact. To keep it simple and not too chemical, all mono-saccharides and few di-, oligo- and poli-saccharides are “reducing“.
The Maillard reaction happens quickly only above 140°C and it causes:
- The superficial and internal browning of baked goods
- A production of many different flavour compounds that alter the final result
Above a certain temperature (for example, above 160°C for sucrose, 150°C for glucose), we talk of caramelization: this is a similar reaction that involves browning and change of flavour. The difference from the Maillard reaction is that caramelization would happen anyway at high temperatures regardless of whether there is interaction with proteins or not.
The more intense the caramelization is, the more the final flavour will change, eventually becoming “bitter” and with a burnt after-taste.
As I mentioned above, the Maillard reaction occurs with reducing sugars such as glucose and fructose. Sucrose is not a reducing sugar, but it can provide the product with the glucose and fructose of which it is made of through the action of acids or enzymes present in the dough/batter; in this case, though, the reaction would be much slower.
Source: “The science of pastry”, by Dario Bressanin